The global warming of the biosphere and its consequent acidification of the oceans is a complex of geophysical, biological and ecological, and sociological phenomena that are all accelerating. There is much that humanity could do to slow that acceleration, and to enact strategies for its own protection from Nature’s escalating assaults on civilization by the grand feedback loop of anthropogenic global warming climate change, but there is really nothing humanity can do to stop it.
Carbon Dioxide Emissions
The anthropogenic emission of carbon dioxide (CO2) — the exhaust fume of economic activity — has increased steadily over the last 270 years, and explosively so for the last 70 years.
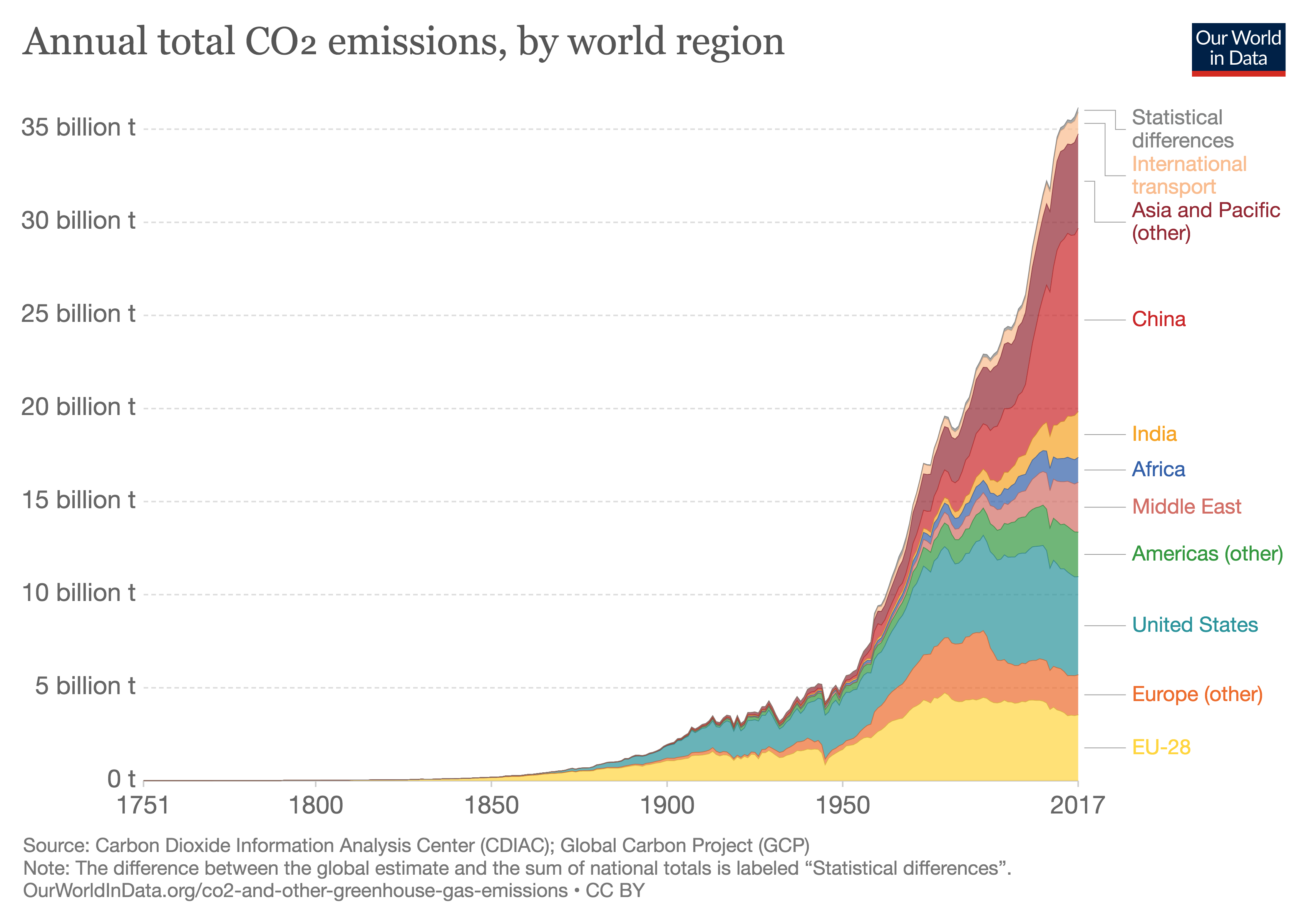
Annual CO2 Emission by Region
Those emissions were 5.28 billion metric tons of CO2 (1 metric ton = 1 tonne = 1000kg = 2,205 lb) in 1950, and 36.15 billion tonnes in 2017 (1 billion tonnes = 1 giga-tonne = 1 Gt). A rough quantitative characterization (analytical fit) to the historical trend of anthropogenic CO2 emissions since the early 20th century is
E = 35.5•[(YEAR-1890)/130]^2, in Gt/year.
The cumulative emissions up to 2017 were 1,540Gt of CO2 (=1.54 trillion tonnes).
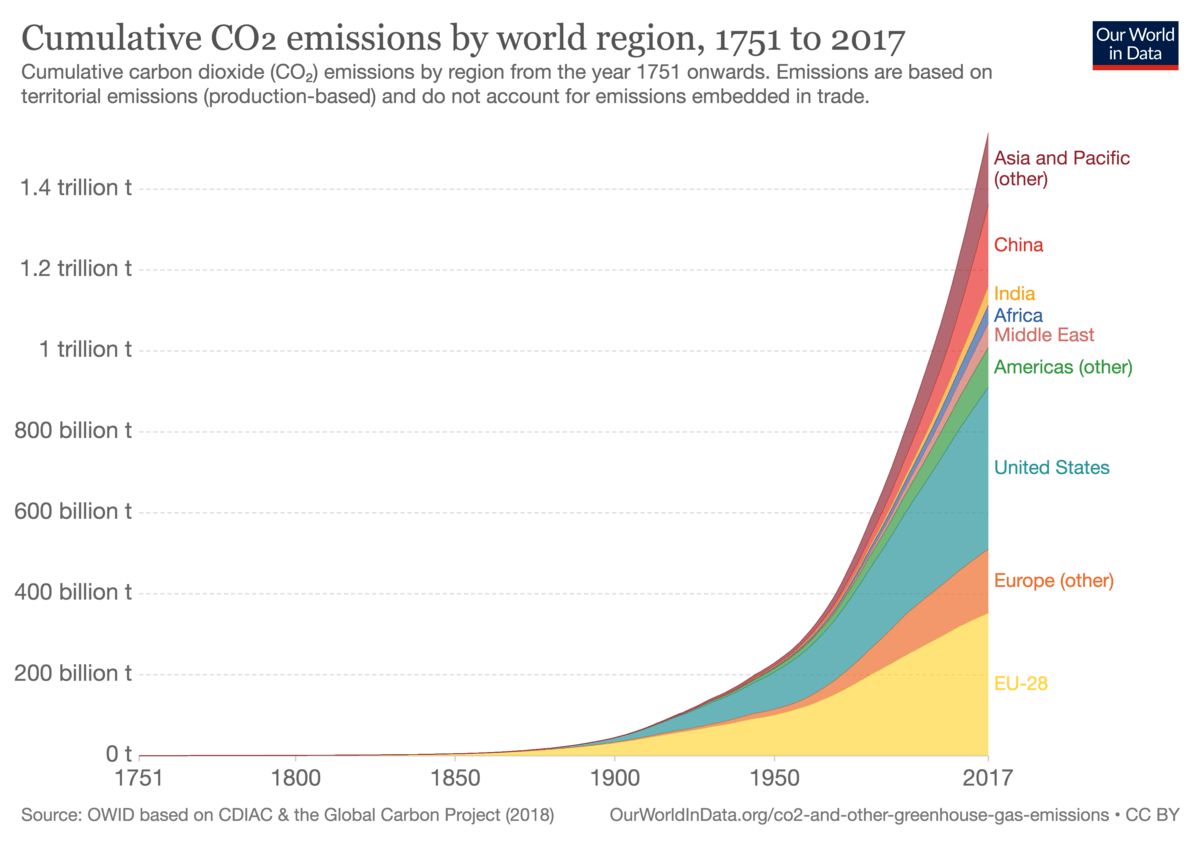
Cumulative CO2 Emissions by Region
Carbon Dioxide in the Oceans
Of the annual CO2 emissions, about 30% are absorbed by the oceans. [1]
A rough quantitative characterization to the historical trend of CO2 absorption by the oceans is
W = 10.4•[(YEAR-1890)/130]^2, in Gt/year.
The cumulative load of anthropogenic CO2 absorbed by the oceans is 450Gt. [2]
According to [3] there are 39,000Gt of carbon currently in the oceans. Since CO2 molecules are 3.667x more massive (‘heavier’) than pure carbon atoms, this represent 143,000Gt of absorbed CO2. The cumulative mass of Earth’s oceans is 1.366GGt (=1.366•10^9 Gt). Thus, the currently absorbed CO2 is in a mass ratio to seawater of 104.7ppmm (=104.7 parts per million by mass). The “ancient” seas (without the 450Gt anthropogenic load of CO2) had 104.4ppmm of CO2.
This seemingly small addition to the CO2 in the oceans has had profound biological and ecological effects, because of the increase of oceanic acidity by 26%. [1], [4] The chemical indicator of acidity used by scientists, pH, has dropped from 8.2 for “ancient” seawater, to 8.1 for present seawater. The pH scale is logarithmic, and its numbers decrease as the solution in question becomes more acidic.
Ocean acidity impedes the ability of shell-forming marine life to produce their protective coverings. With increased ocean acidity, even the shell structures in existence are eroded. These effects make it more difficult for shell-forming marine life to survive, and as many of these life-forms are small (part of the plankton) they are essential foods at the base of the marine food chain. So the ultimate concern about escalating oceanic acidity is the potential for a collapse of marine life. One estimate of the CO2 concentration needed for “ocean death” by acidification is 400ppmm to 500ppmm. [3]
This implies that 400,000Gt to 540,000Gt more of CO2 would have to be deposited into the oceans; a task that would require 38,000 years to 52,000 years of anthropogenic emissions at the current rate (10.4Gt/year into the oceans). However, “ocean dying” is plainly evident with the current quantity of absorbed CO2, and it will only get worse at an accelerating pace as more CO2 is emitted by civilization.
The chemistry of ocean acidification is as follows. [1]
CO2 + H2O + CO3 —> 2HCO3
Carbon dioxide plus water plus a carbonate ion react to form 2 bicarbonate ions. This process occurs in three steps:
CO2 + H2O —> H2CO3
Carbon dioxide plus water form carbonic acid, which is a weakly bound molecule.
H2CO3 —> H(+) + HCO3(-)
Carbonic acid breaks up into a hydrogen ion and a bicarbonate ion.
H(+) + CO3(2-) —> HCO3(-)
The hydrogen ions liberated in the previous reaction find carbonate ions floating in seawater, and combine into bicarbonate ions. The net result is two bicarbonate ions in the seawater solution.
Shell-forming marine life capture carbonate ions, CO3(2-), to combine them with calcium into calcium carbonate, CaCO3, to form their pearls and seashells. Extracting the needed carbonate by breaking apart bicarbonate ions, instead of just collecting free-floating carbonate ions, is more energy intensive and thus a frustration of the shell-forming biology of so much marine life. So, ocean acidification by CO2 removes some of the stores of a formally available free-floating carbonate ions from the reach of shell-forming marine life.
That acidity, a function of the liberated hydrogen ions, H(+), can also dissolve existing shells. [5]
CaCO3 + 2H(+) —> Ca(2+) + CO2 + H2O
Calcium carbonate (shells) plus hydrogen ions react, dissolving the shell, into free-floating calcium ions plus absorbed carbon dioxide gas plus water.
The Rate of Global Warming is Accelerating
From what has been described up to this point, in conjunction with my previous modeling, I calculate the following tabulated results.
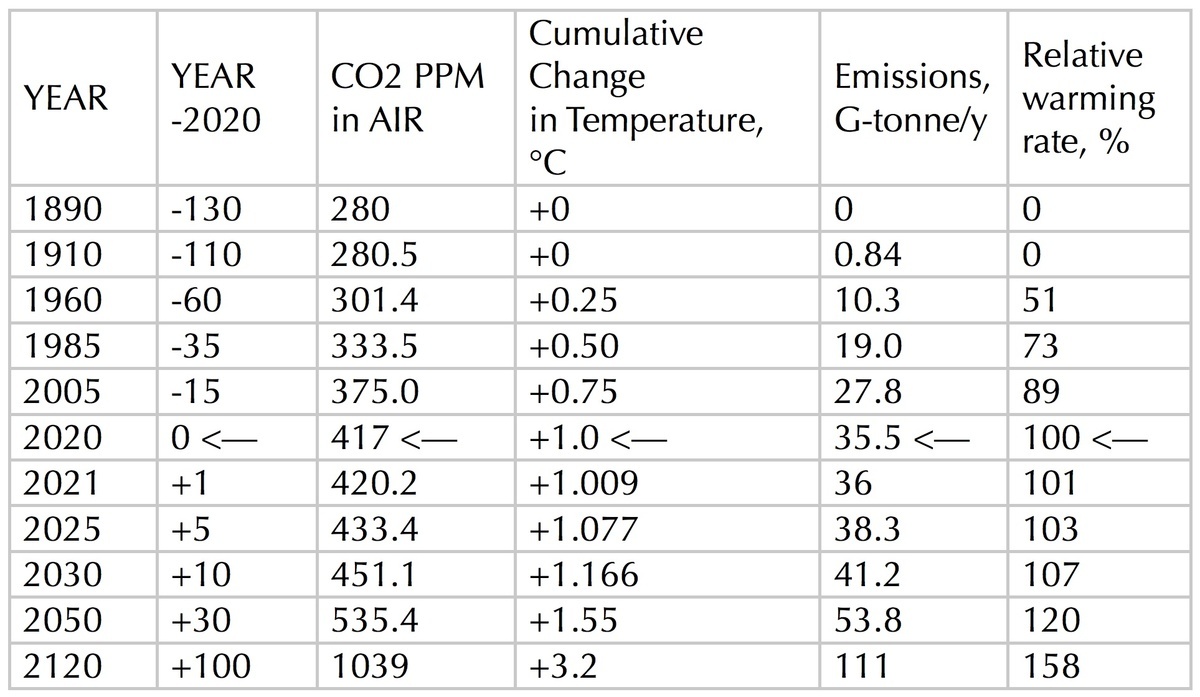
TABLE, Relative Warming Rate, %
Note that the rate at which global temperature is increasing is accelerating, as is the rate of global warming (the Watts absorbed by the biosphere each year). Also note that entries after 2020 are necessarily projections, and are based on the assumption of existing trends (and the analytical formulas fitted to them) continuing. The entries listed for the year 2020 are pointed out to show that earlier entries are backed by data, and later entries are projections; and to note that rate of global warming for any year listed is shown as a ratio to its rate for year 2020.
The Rate of Ocean Acidification is Accelerating
From what has been described up to this point, I calculate the following tabulated results.
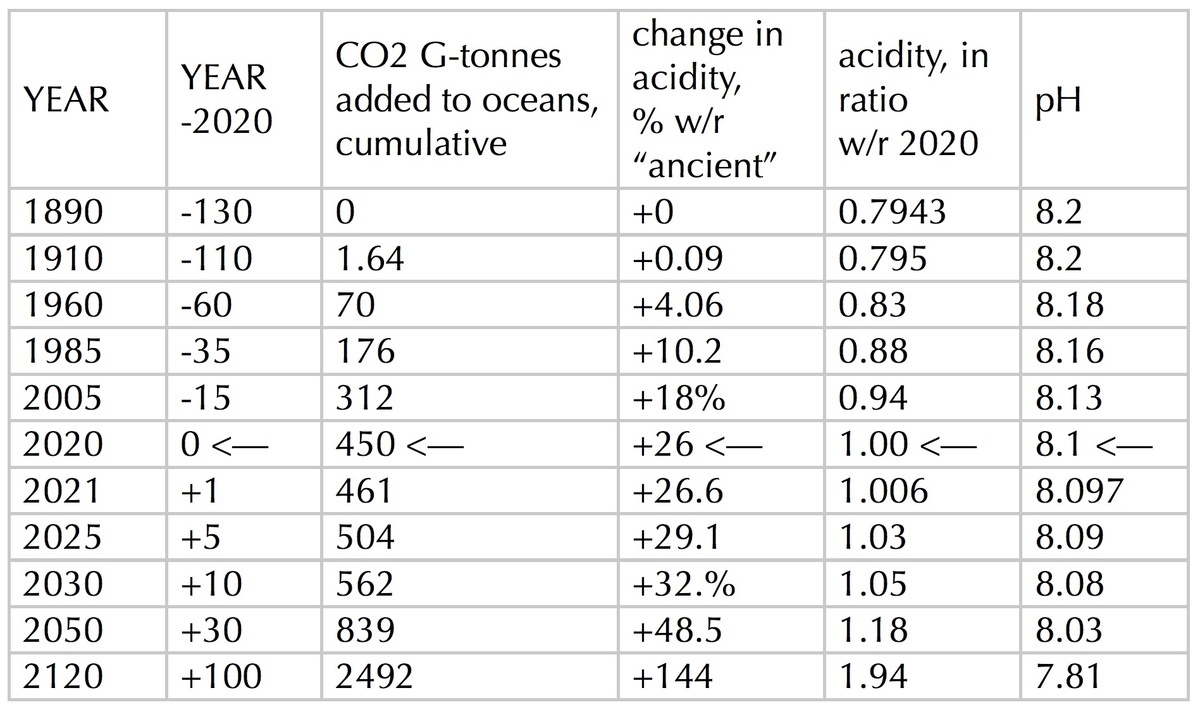
TABLE, Ocean Acidification
As in the first table, entries up to year 2020 are backed by data, while those after year 2020 are projections. Today’s oceans are 26% more acidic than the oceans of the late 19th century. An alternative comparison is that the oceans of the late 19th century were only 79% as acidic as they are today. If the current trend — of annually increasing anthropogenic CO2 emissions — continues to the end of the 21st century, then the oceans would be 144% (2.44x) more acidic than in the late 19th century; or, equivalently, almost twice as acidic as they are today. Those future acidic oceans at pH=7.8 would reproduce conditions during the middle Miocene, 14 to 17 million years ago, when the Earth was several degrees warmer and a major extinction event was occurring. [1], [4]
“Fixing” Global Warming
I see no possibility of a technical “miracle” to fix global warming; something like an anti-global-warming planetary vaccine, making civilization safe to continue with capitalism.
The CO2 in the biosphere is an extremely dilute mass within enormous masses and expanses of air and water. Removing the anthropogenic excesses of CO2 from the air and the oceans would require the filtration of an immense bulk of matter. Processes of such filtration would require immense quantities of energy, to pump and chemically “strain.” Even if we were able to generate sufficient quantities of energy to power such processes, I cannot imagine that generation to be free of CO2 emissions that would exceed whatever quantity of CO2 was strained out of the biosphere. So, I see such ideas of “technical fixes” as fantasies of the perpetual motion machine variety, and obviated by the 2nd Law of Thermodynamics (specifically, as it applies to reversing the process of diffusion).
The only lever I see humanity having with which to influence the pace of global warming is the degree of its restraint in emitting CO2 in the first place. There is no more energy-efficient counter-warming strategy we can devise. The most effective protective armor that can be devised to shield people from the potential harm that playing Russian Roulette can inflict is to not shoot themselves in the head in the first place.
The energy that we do generate and use to counteract the negative effects of global warming (not just to humans, but to thousands of other species) is best spent in transforming our societies and civilization for maximal mutual assistance and solidarity, and minimal competitive tribalism. Some of that energy would go into physical constructions to shield people from floods, inundation, excessive heat and drought; and some of that energy would go into civic arrangements for sheltering, feeding, healthcare and economic stability of all individuals, and the resettlement of those displaced by loss of habitat: by the loss of coastal land to the rising of sea level, and the loss of living space in continental interiors because of the onset of unlivable heat and loss of water.
Essential to the energy efficiency of both devising and implementing such counter-warming social transformations, it is necessary to stop wasting energy on activities without intrinsic social benefits. Specifically, we, worldwide — but most especially among the 10% wealthiest of Earth’s people, who produce 49% of anthropogenic CO2 emissions [6] — need to abandon every trace of profligate CO2-spewing lifestyles enabled by competitive and exclusionary capitalism and its plethora of bigotries, to instead join cooperatively in World Socialism without consumerist economics nor tribal animosities.
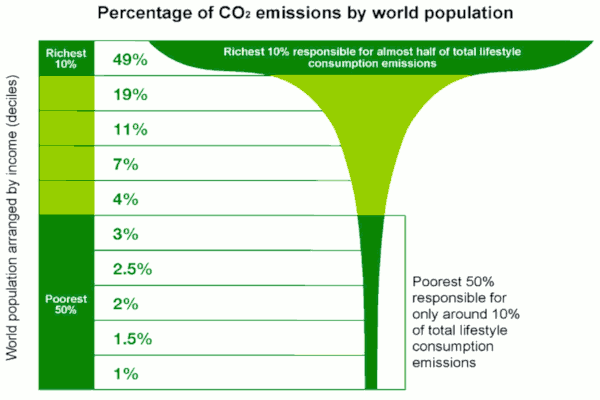
Percentage of CO2 Emissions by World Population (Oxfam)
Planet Earth is the loveliest jewel we know of in the entire Universe. If we treated it as such, and each other as part of the sparkle of that gem, we would experience lives in an actual Paradise, regardless of how challenging global warming made our existence.
Notes
[1] Ocean Acidification
https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification
[2] Cumulative anthropogenic CO2 absorbed by oceans is 450Gt
Previously, I showed that 1,090Gt of CO2 currently resides in the atmosphere; thus 1,540Gt – 1,090Gt = 450Gt. [450Gt/1,540Gt]•100% = 29.2%.
[3] Ocean storage of carbon dioxide
https://en.wikipedia.org/wiki/Ocean_storage_of_carbon_dioxide
[4] A primer on pH
https://pmel.noaa.gov/co2/story/A+primer+on+pH
[5] Calcium carbonate
https://en.wikipedia.org/wiki/Calcium_carbonate
[6] Image: Percentage of CO2 emissions by world population, was produced by OXFAM.